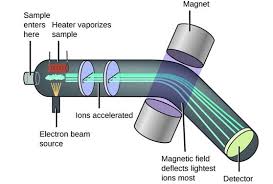
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions.
Principle:
Mass Spectrometer(MS) generates multiple ions from the sample under investigation, it then separates them according to their specific mass-to-charge ratio (m/z), and then records the relative abundance of each ion type.
Every mass spectrometer consists of at least these three components:
1.Ionization Source 2.Mass Analyzer 3.Ion Detection System
Some Applications:
Mass spectrometry has both qualitative and quantitative uses. These include identifying unknown compounds, determining the isotopic composition of elements in a molecule, and determining the structure of a compound by observing its fragmentation. Other uses include quantifying the amount of a compound in a sample or studying the fundamentals of gas phase ion chemistry (the chemistry of ions and neutrals in a vacuum). Mass spectrometers are also widely used in space missions to measure the composition of plasmas.
Mass spectrometry is an important method for the characterization and sequencing of proteins. The two primary methods for ionization of whole proteins are electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI).
Pharmacokinetics is often studied using mass spectrometry because of the complex nature of the matrix (often blood or urine) and the need for high sensitivity to observe low dose and long time point data.
Some important applications in the field of gaining space knowledge.This instrument analyzed atmospheric samples along its descent trajectory and was able to vaporize and analyze samples of Titan’s frozen, hydrocarbon covered surface once the probe had landed. These measurements compare the abundance of isotope(s) of each particle comparatively to earth’s natural abundance.
Mass spectrometry in the nose of a NASA WB-57
Some disadvantages:
a few of the disadvantages of the method is that it often fails to distinguish between optical and geometrical isomers and the positions of substituent in o-, m- and p- positions in an aromatic ring. Also, its scope is limited in identifying hydrocarbons that produce similar fragmented ion.
Some advances in mass spectrometry:
New mass spectrometry (MS) methods, collectively known as data independent analysis and hyper reaction monitoring, have recently emerged. These methods hold promises to address the shortcomings of data-dependent analysis and selected reaction monitoring (SRM) employed in shotgun and targeted proteomics, respectively. They allow MS analyses of all species in a complex sample indiscriminately, or permit SRM-like experiments conducted with full high-resolution product ion spectra, potentially leading to higher sequence coverage or analytical selectivity. These methods include MSE, all-ion fragmentation, Fourier transform-all reaction monitoring, SWATH Acquisition, multiplexed MS/MS, pseudo-SRM (pSRM) and parallel reaction monitoring (PRM). In this review, the strengths and pitfalls of these methods are discussed and illustrated with examples. In essence, the suitability of the use of each method is contingent on the biological questions posed. Although these methods do not fundamentally change the shape of proteomics, they are useful additional tools that should expedite biological discoveries.